CL0503
Description
BACKGROUND The selectin family of adhesion molecules mediates the initial attachment of leukocytes to venular endothelial cells before their firm adhesion and diapedesis at sites of tissue injury and inflammation. The selectin family consists of three closely related cell-surface molecules with differential expression by leukocytes (L-selectin), platelets (P-selectin), and vascular endothelium (E- and P-selectin). The selectins have characteristic extracellular regions composed of an amino-terminal lectin domain that binds a carbohydrate ligand, an epidermal growth factor-like domain, and two to nine short repeat units homologous to domains found in complement binding proteins. In contrast to most other adhesion molecules, selectin function is restricted to leukocyte interactions with vascular endothelium. Multiple studies indicate that the selectins mediate neutrophil, monocyte, and lymphocyte rolling along the venular wall. The generation of selectin-deficient mice has confirmed these findings and provided further insight into how the overlapping functions of these receptors regulate inflammatory processes. Selectin-directed therapeutic agents are now proven to be effective in blocking many of the pathological effects resulting from leukocyte entry into sites of inflammation.1
L-selectin (CD62L) is a cell adhesion molecule consisting of a large, highly glycosylated, extracellular domain, a single spanning transmembrane domain and a small cytoplasmic tail. It is expressed on most leukocytes and is involved in their rolling on inflamed vascular endothelium prior to firm adhesion and transmigration. It is also required for the constitutive trafficking of lymphocytes through secondary lymphoid organs. Like most adhesion molecules, L-selectin function is regulated by a variety of mechanisms including gene transcription, post-translational modifications, association with the actin cytoskeleton, and topographic distribution. In addition, it is rapidly downregulated by proteolytic cleavage near the cell surface by ADAM-17 (TACE) and at least one other “sheddase”. This process of “ectodomain shedding” results in the release of most of the extracellular portion of L-selectin from the cell surface while retaining the cytoplasmic, transmembrane, and eleven amino acids of the extracellular domain on the cell.2 In addition, The activation of leukocytes by bacterial cell-wall lipopolysaccharide contributes to the pathogenesis of septic shock. It was suggested that in neutrophils, and possibly other leukocytes, L-selectin can act as a low-affinity lipopolysaccharide receptor. Inhibitors of L-selectin may therefore be of therapeutic value in treating this life-threatening condition.3
L-selectin (CD62L) is a cell adhesion molecule consisting of a large, highly glycosylated, extracellular domain, a single spanning transmembrane domain and a small cytoplasmic tail. It is expressed on most leukocytes and is involved in their rolling on inflamed vascular endothelium prior to firm adhesion and transmigration. It is also required for the constitutive trafficking of lymphocytes through secondary lymphoid organs. Like most adhesion molecules, L-selectin function is regulated by a variety of mechanisms including gene transcription, post-translational modifications, association with the actin cytoskeleton, and topographic distribution. In addition, it is rapidly downregulated by proteolytic cleavage near the cell surface by ADAM-17 (TACE) and at least one other “sheddase”. This process of “ectodomain shedding” results in the release of most of the extracellular portion of L-selectin from the cell surface while retaining the cytoplasmic, transmembrane, and eleven amino acids of the extracellular domain on the cell.2 In addition, The activation of leukocytes by bacterial cell-wall lipopolysaccharide contributes to the pathogenesis of septic shock. It was suggested that in neutrophils, and possibly other leukocytes, L-selectin can act as a low-affinity lipopolysaccharide receptor. Inhibitors of L-selectin may therefore be of therapeutic value in treating this life-threatening condition.3
REFERENCES
1. Rosen, S.D. et al: Ann. Rev. Immunol. 22:129-56, 2003
2. Smalley, D.M. & Ley, K.: J. Cell. Mol. Med. 9:255-66, 2005
3. Malhotra, R. & Bird, B.I.: Chem. Biol. 4:543-7, 1997
2. Smalley, D.M. & Ley, K.: J. Cell. Mol. Med. 9:255-66, 2005
3. Malhotra, R. & Bird, B.I.: Chem. Biol. 4:543-7, 1997
Products are for research use only. They are not intended for human, animal, or diagnostic applications.
Details
Cat.No.: | CL0503 |
Target Protein Species: | Human |
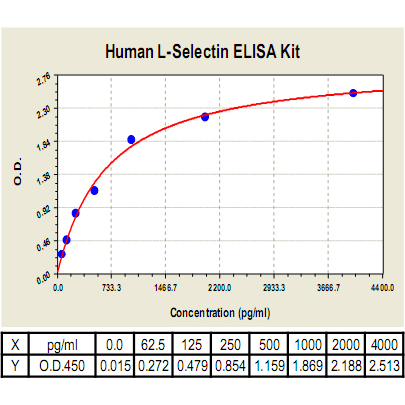
Range: | 62.5pg/ml-4000pg/ml |
Specificity: | No detectable cross-reactivity with any other cytokine. |
Storage: | Store at 4°C. Use within 6 months. |
ELISA Kits are based on standard sandwich enzyme-linked immunosorbent assay technology. Freshly prepared standards, samples, and solutions are recommended for best results.
Products
| Product | Size | CAT.# | Price | Quantity |
|---|---|---|---|---|
| Human L-Selectin ELISA Kit: Human L-Selectin ELISA Kit | Size: 96 wells | CAT.#: CL0503 | Price: $500.00 |