Product Sheet CP10189
Description
BACKGROUND Nucleophosmin (NPM; also known as B23, NO38, or numatrin) is an abundant and ubiquitously expressed nucleolar phosphoprotein which has been implicated in ribosome biogenesis. Indeed, NPM binds nucleic acids, associates with maturing preribosomal ribonucleoprotein particles, and possesses intrinsic RNase activity and its down-regulation retards the processing of pre-rRNA. NPM acts as a molecular chaperone and shuttles between the nucleus and cytoplasm, suggesting that it may also prevent aggregation of nucleolar proteins and facilitate nuclear export.1
NPM has the additional property of regulating cell proliferation. Down-regulation of NPM in normal or immortalized cells delays cell division or induces apoptosis. NPM has also been implicated in the acute response of mammalian cells to environmental stresses, particularly DNA-damaging agents. UV light, for example, induces up-regulation and nuclear relocalization of NPM that, under these experimental conditions, stimulates DNA repair and reduces apoptosis.
NPM has the additional property of regulating cell proliferation. Down-regulation of NPM in normal or immortalized cells delays cell division or induces apoptosis. NPM has also been implicated in the acute response of mammalian cells to environmental stresses, particularly DNA-damaging agents. UV light, for example, induces up-regulation and nuclear relocalization of NPM that, under these experimental conditions, stimulates DNA repair and reduces apoptosis.
NPM is part of a high-molecular-weight complex and physically interacts with many cellular proteins, including p53, Mdm2, and Arf. NPM increases the stability and transcriptional activity of p53, likely through multiple mechanisms, including its chaperone activity on p53, inhibition of the Mdm2 ubiquitin-ligase activity, and competition with p53-Mdm2 binding. Other laboratories, however, using different cell systems and experimental approaches, have reported an inhibitory effect of NPM on phosphorylation of p53 and attenuation of its transcriptional effects. The Arf-NPM interaction seems to be critical in regulating the stability of both proteins. Arf, in fact, induces polyubiquitination and degradation of NPM and inhibits its effects on ribogenesis . NPM, instead, protects Arf from degradation and, surprisingly, antagonizes its ability to inhibit cell division.2 The effects of NPM on the Arf-Mdm2-p53 tumor suppressor pathway, as well as its activities on ribogenesis, cell division, and survival, might be relevant for the role of NPM in human cancer. NPM, in fact, is frequently overexpressed in tumors of different origin and is the most frequent target of genetic alterations in hematopoietic tumors: it is rearranged with the ALK gene in the majority of anaplastic large-cell lymphomas and is mutated in 35% of acute myeloid leukemias.3
REFERENCES
1. Inder, K.L. et al: Commun Integr Biol. 3:188-90, 2010
2. Colombo, E. et al: Mol. Cell. Biol. 25:8874-86, 2005
3. Grisendi, S. et al: Nature Rev. Cancer 6:493-505, 2006
2. Colombo, E. et al: Mol. Cell. Biol. 25:8874-86, 2005
3. Grisendi, S. et al: Nature Rev. Cancer 6:493-505, 2006
Products are for research use only. They are not intended for human, animal, or diagnostic applications.
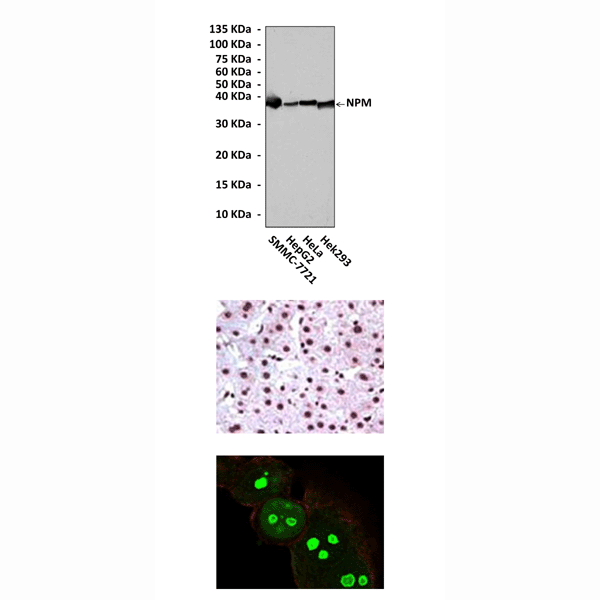
(Click to Enlarge) Top: Western Blot detection of NPM proteins in various cell lysates using NPM Antibody. Middle: This antibody stains paraffin-embedded human hepatoma tissue in immunohistochemical analysis. Bottom: It also stains HeLa cells in confocal immunofluorescent testing (NPM Antibody: Green; Actin filament: Red).
Details
Cat.No.: | CP10189 |
Antigen: | Purified recombinant human NPM fragments expressed in E. coli. |
Isotype: | Mouse IgG |
Species & predicted species cross- reactivity ( ): | Human, Monkey |
Applications & Suggested starting dilutions:* | WB 1:1000 IP n/d IHC 1:200 ICC 1:200 FACS n/d |
Predicted Molecular Weight of protein: | 38 kDa |
Specificity/Sensitivity: | Detects endogenous NPM proteins without cross-reactivity with other family members. |
Storage: | Store at -20°C, 4°C for frequent use. Avoid repeated freeze-thaw cycles. |
*Optimal working dilutions must be determined by end user.
Products
| Product | Size | CAT.# | Price | Quantity |
|---|---|---|---|---|
| Mouse Nucleophosmin Antibody: Mouse Nucleophosmin Antibody | Size: 100 ul | CAT.#: CP10189 | Price: $354.00 |
