Product Sheet CL0461
Description
BACKGROUND MMPs (matrix metalloproteinases) are zinc-dependent endopeptidases that are mainly involved in turnover and proteolytic degradation of the extracellular matrix and are believed to play a role in tissue remodeling in a number of normal and pathological conditions. MMPs may play a key role in physiologic and pathologic remodeling of tissues, including embryogenesis and tissue morphogenesis, wound repair, inflammatory diseases, cancerogenesis, etc. These multiple actions of MMPs are due to their ability to degrade extracellular matrices, basement membranes, and other proteins. So far, more than 25 members of the MMP family have been identified, and they can be divided into four groups on the basis of their domain structure, substrate specificity and cellular localization. These four groups are collagenases (MMP-1, -8 and -13), stromelysins (MMP-3, -7, -10, -11 and -12), gelatinases (MMP-2 and -9) and membrane-type MMPs.1
MMP-3 is a 45 kDa protein with a wide range of connective tissue substrates, including proteoglycans, gelatin, laminin, fibronectin and collagen (types III, IV and IX). In addition, MMP-3 can stimulate the other enzymes in the MMPs group, such as MMP-1, MMP-7, MMP-8, MMP-9 and MMP-13. This stimulation increases biochemical substance degradation, including degradation of type II collagen, the most important type of collagen in the extracellular matrix (ECM).2 Moreover, MMP-3 can activate several serpin-type serine proteinase inhibitors. In addition, MMP-3 can release a number of cell surface molecules, including E-cadherin, a known contributor to cancer development. Thus, MMP-3 exhibits a number of activities that would make it a particularly good tumor promoter.3 MMP-3 is produced by various types of cells; fibroblast, smooth muscle cells, and macrophages. Similar to other secreted proteinases, the catalytic activity of MMP-3 is regulated at four stages, namely transcription, compartmentalization, proenzyme activation and enzyme inactivation by natural inhibitors. The production of MMP-3 is mainly regulated at the level of transcription. The expression of MMP-3 in unstimulated cells is low, but it is induced by a variety of extracellular stimuli, including mitogenic growth factors, cytokines and contact with the extracellular matrix. Owing to the similarities in structure across the MMP family, it is important to distinctively control transcription according to cell type or different stimulators. Several transcription factors, including NF-kappaB (nuclear factor kappaB), the transcription factor ETS-1 (E26 transformation-specific-1) and AP-1 (activator protein-1), are reported to be involved in the transcriptional induction of MMP-3. MMP-3 is known to mediate the release of macrophage chemotactic activity from chondrocytes. In addition, serum MMP-3 level is a clinically useful maker for predicting joint destruction and for disease activity in rheumatoid arthritis (RA).4
MMP-3 is a 45 kDa protein with a wide range of connective tissue substrates, including proteoglycans, gelatin, laminin, fibronectin and collagen (types III, IV and IX). In addition, MMP-3 can stimulate the other enzymes in the MMPs group, such as MMP-1, MMP-7, MMP-8, MMP-9 and MMP-13. This stimulation increases biochemical substance degradation, including degradation of type II collagen, the most important type of collagen in the extracellular matrix (ECM).2 Moreover, MMP-3 can activate several serpin-type serine proteinase inhibitors. In addition, MMP-3 can release a number of cell surface molecules, including E-cadherin, a known contributor to cancer development. Thus, MMP-3 exhibits a number of activities that would make it a particularly good tumor promoter.3 MMP-3 is produced by various types of cells; fibroblast, smooth muscle cells, and macrophages. Similar to other secreted proteinases, the catalytic activity of MMP-3 is regulated at four stages, namely transcription, compartmentalization, proenzyme activation and enzyme inactivation by natural inhibitors. The production of MMP-3 is mainly regulated at the level of transcription. The expression of MMP-3 in unstimulated cells is low, but it is induced by a variety of extracellular stimuli, including mitogenic growth factors, cytokines and contact with the extracellular matrix. Owing to the similarities in structure across the MMP family, it is important to distinctively control transcription according to cell type or different stimulators. Several transcription factors, including NF-kappaB (nuclear factor kappaB), the transcription factor ETS-1 (E26 transformation-specific-1) and AP-1 (activator protein-1), are reported to be involved in the transcriptional induction of MMP-3. MMP-3 is known to mediate the release of macrophage chemotactic activity from chondrocytes. In addition, serum MMP-3 level is a clinically useful maker for predicting joint destruction and for disease activity in rheumatoid arthritis (RA).4
REFERENCES
1. Chakraborti, S. et al: Mol Cell Biochem. 253:269-85, 2003
2. Lin, P.M. et al: Osteoarthritis and Cartilage, 12:485-96, 2004
3. Sternlicht,M.D. et al: Oncogene 19:1102-13, 2000
4. Shinozaki, M. et al: Mod Rheumatol. 17:403-8, 2007
2. Lin, P.M. et al: Osteoarthritis and Cartilage, 12:485-96, 2004
3. Sternlicht,M.D. et al: Oncogene 19:1102-13, 2000
4. Shinozaki, M. et al: Mod Rheumatol. 17:403-8, 2007
Products are for research use only. They are not intended for human, animal, or diagnostic applications.
Details
Cat.No.: | CL0461 |
Target Protein Species: | Human |
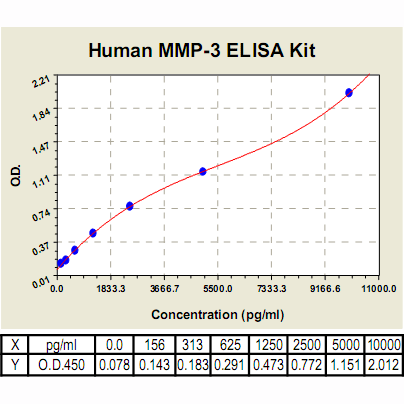
Range: | 156pg/ml – 10000pg/ml |
Specificity: | Cross-reactivates with MMP-10 approximately 2%, and no detectable cross-reactivity with other MMPs. |
Storage: | Store at 4°C. Use within 6 months. |
ELISA Kits are based on standard sandwich enzyme-linked immunosorbent assay technology. Freshly prepared standards, samples, and solutions are recommended for best results.
Products
| Product | Size | CAT.# | Price | Quantity |
|---|---|---|---|---|
| Human MMP-3 ELISA Kit: Human Matrix Metalloproteinase-3 ELISA Kit | Size: 96 Wells | CAT.#: CL0461 | Price: $500.00 |