Human Induced Pluripotent Stem Cells: HiPSC
Description
|
Jump to HiPSC Links |
Integration-free HiPSC. Our HiPSC are generated with the RNA-based Sendai virus to deliver reprogramming factors to donor skin fibroblasts. Since the virus does not go through a DNA phase, its genetic material and transgenes do not integrate into the host cell genome. HiPSC are validated for viability, karyotype, pluripotency, plating efficiency, morphology, passage number and lack of contamination.
HiPSC-derived Neural Stem Cells (L) and Neurons (R). i-HNSC stained w/ Nestin (neural stem cell marker, green), SOX 2 (stem cell marker, red) & DAPI (nuclear stain, blue). SOX2 & DAPI nuclear co-localization yields purple (Left). Video: Human iPSC-Derived Neurons establish mature, synchronized neuronal network. In real time, Multi-electrode array (MEA) shows optimal electrophysiological activity, which can be modulated by neurotransmitters or small compounds (Right).
HiPSC-derived Cardiomyocytes (i-HCM) plated onto a flat culture surface pulsate in vitro (L), while iHCM printed into 3D heart tissue using a Cyfuse Regenova also beat (R)
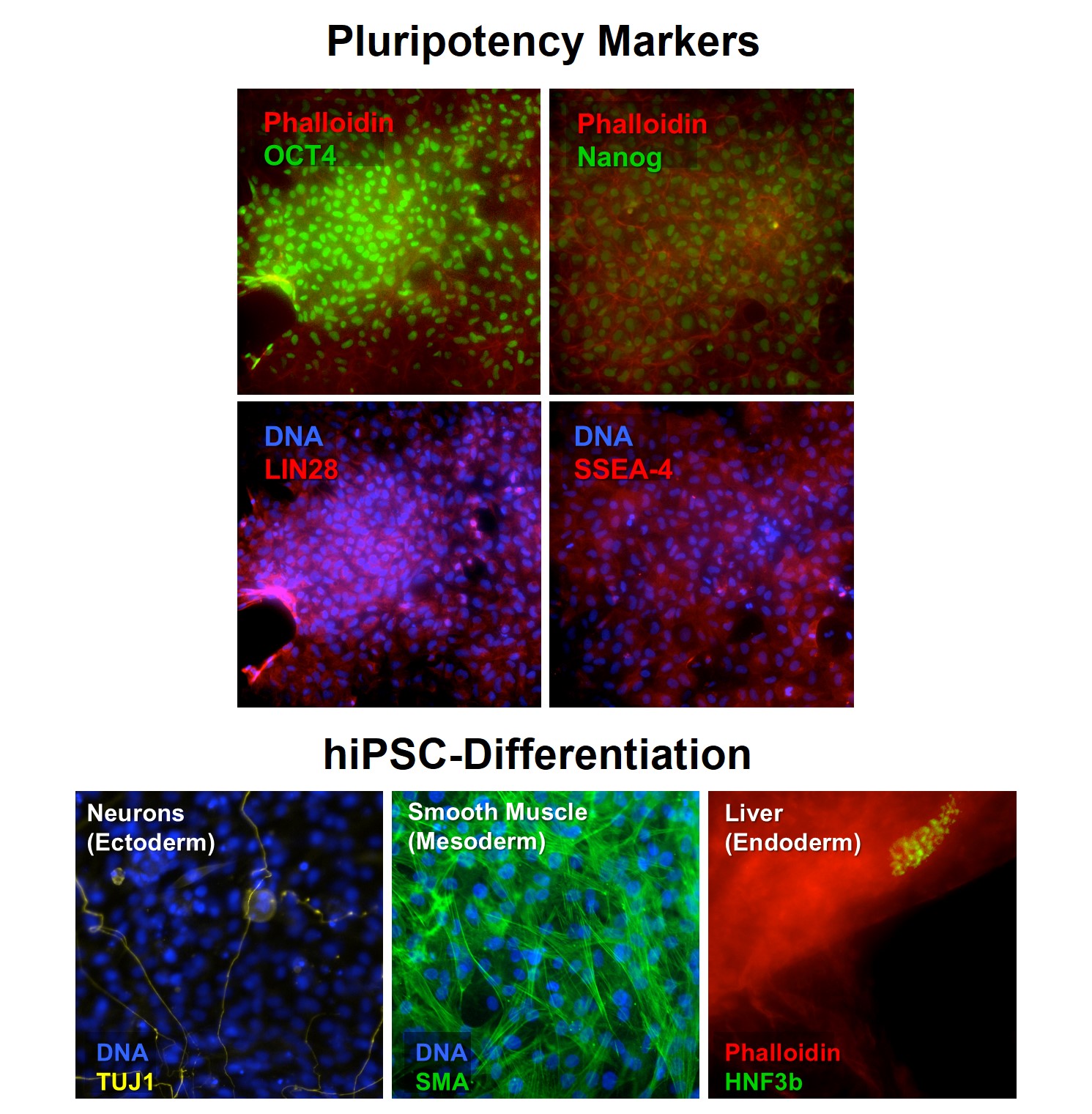
Reprogramming triggers a cascade of evident changes in the host cells that are recognizable morphologically and through a combination of markers and pluripotency assays. Our HiPSCs display classic pluripotent stem cell morphology, with a high nucleus to cytoplasm size ratio, as well as they are amenable to be cultivated in serum-free media, independent of feeder cells and of feeder-conditioned media as colonies or high density monolayers. hiPSCs are also evaluated for the presence of karyotypic abnormalities. Confirmation of pluripotency is performed through the analysis of expression of several established independent pluripotency markers. Unguided differentiation confirms HiPSC ability to generate cell derivatives of tissues arising from the three embryonic layers.
Cell Characterization. Post-thawing viability of HiPSCs is typically higher than 70%, and HiPSC have demonstrated coherent pluripotent behavior over more than 60 passages. Although it is in theory possible to propagate HiPSCs indefinitely, HiPSC subculturing over passages higher than is usually not recommended, as the chances of karyotypic abnormalities increase. HiPSCs are also tested to ensure absence of microorganism contaminants.
For Research Use Only – Not for Human or Clinical Applications
Details
| Cryopreserved HiPSC | ||
| Source | Neonatal Male (iPS11-10) or Adult Female (iPS12-10) human dermal fibroblasts (single donor), reprogrammed w/ replication-deficient Sendai virus | |
|---|---|---|
| QC | No bacteria, yeast, fungi, mycoplasma, virus (Trace Sendai virus possible) | |
| CofA | Donor, gender, tissue source, disease status, cell viability (post-thaw), plating efficiency, passage #, karyotype, pluripotency | |
| Cryovial | 1,000,000 HiPSC (low passage) frozen in serum-free HiPSC Freezing Medium | |
| Medium | Cells manufactured, maintained in serum-free, feeder-free culture conditions | |
| Doublings | At least 60 w/out lost morphology, phenotype | |
| Storage | Liquid Nitrogen | |
| Bioassay | PSC morphology, growth behavior when grown in pluripotency conditions | |
| Applications | Non-commercial research use only (RUO), not for human or clinical use | |
| HiPSC Total Kits | (See HiPSC Medium for details) | ||||
|---|---|---|---|---|---|
| Component | Vol | Item No. | oC | ||
| Cryopreserved | Cell Ampoule | 1ml | iPS11-10 or iPS12-10 | LN2 | |
| Growth Medium | Kit (015XFK-500) | 500ml 10ml | Basal Med (014-500) Gr Supp (015XF-GS) | 4C -20C | |
| Coating Solution, Xeno-Free | 25ml | 126XF-25 | -20C | ||
| Dissociation | Kit (091K) | 50ml 25ml | PBS (060-50) Dissoc Soln (076-25) | 4C 4C |
Resources
FAQs
Need More Help?
Visit our comprehensive FAQ page for detailed answers to common questions.
Need More Help?
Visit our comprehensive FAQ page for detailed answers to common questions.
Primary Cell FAQs