MSDS Cryopreserved Cells
Instructions HBEpC Normal
Cell Apps Flyer Epithelial Cells
5 Important Cell Culture Rules
Cell Apps Poster Primary Cells
Cell Applications Inc Brochure
Description
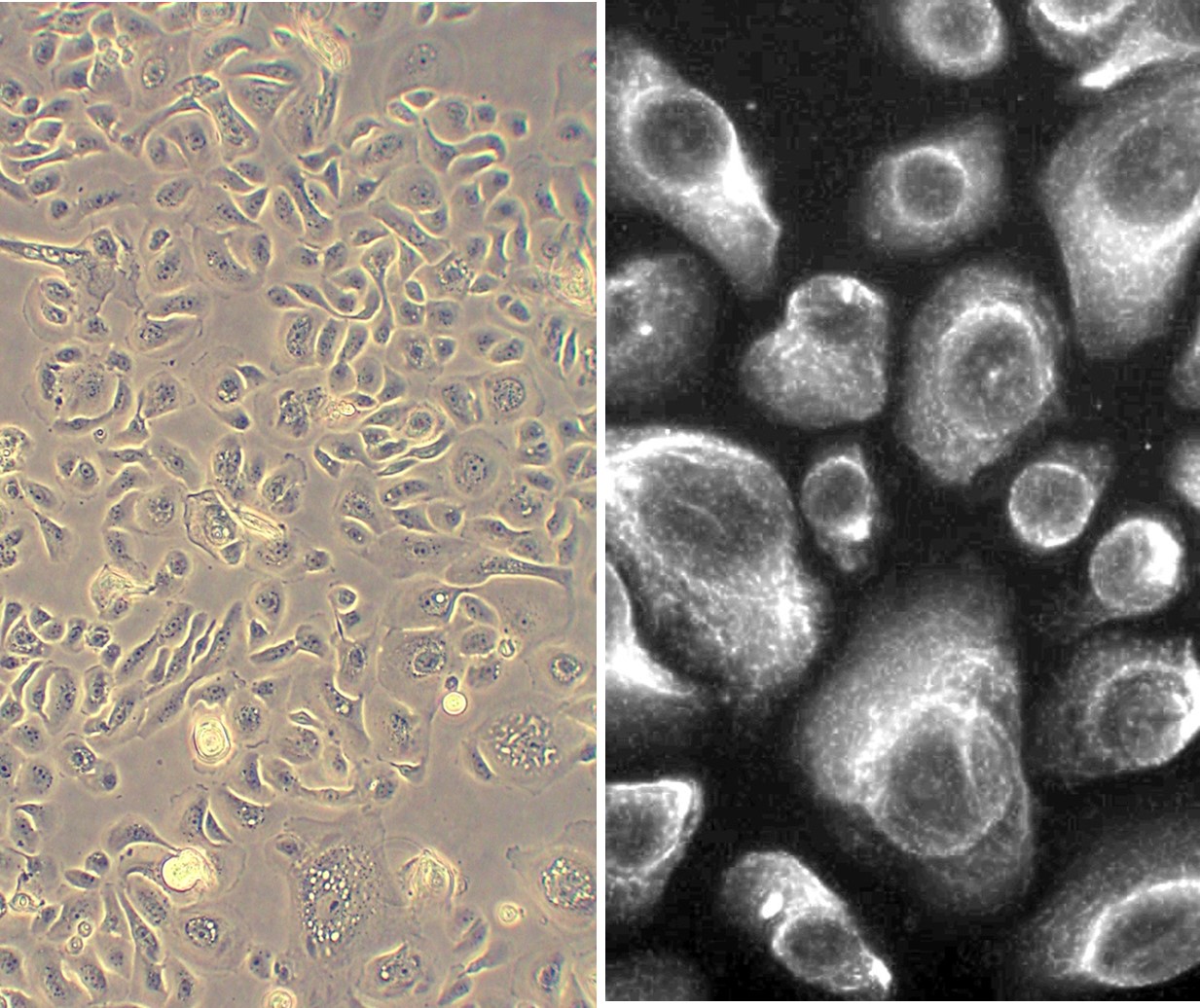 Human Bronchial Epithelial Cells (HBEpC) provide an excellent model system to study all aspects of epithelial function and disease, particularly those related to airway viral infections, as well as tissue repair mechanisms, signaling changes and potential treatments relevant to lung injuries, mechanical and oxidative stress, inflammation, pulmonary diseases and smoking. When grown on inserts and provided with the liquid/air interface, HBEpC can differentiate into a pseudostriated epithelium and serve as a more physiological 3D tissue model for in vitro studies. The HBEpC shown here were cultured (L) and immunolabeled for cytokeratin 18 (R).
Human Bronchial Epithelial Cells (HBEpC) provide an excellent model system to study all aspects of epithelial function and disease, particularly those related to airway viral infections, as well as tissue repair mechanisms, signaling changes and potential treatments relevant to lung injuries, mechanical and oxidative stress, inflammation, pulmonary diseases and smoking. When grown on inserts and provided with the liquid/air interface, HBEpC can differentiate into a pseudostriated epithelium and serve as a more physiological 3D tissue model for in vitro studies. The HBEpC shown here were cultured (L) and immunolabeled for cytokeratin 18 (R). Below, HBEpC from Cell Applications are stained with ACE2 and KRT5. Ongoing research suggests ACE2 is the cellular receptor utilized by SARS-CoV-2, the coronavirus that causes COVID-19, for cell entry.
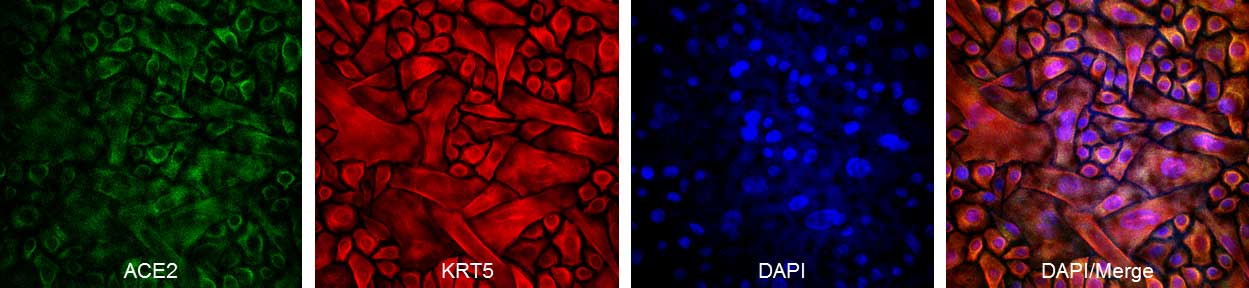
HBEpC from Cell Applications, Inc. have been used to examine:
- Activation, expression and production of genes, kinases and signaling pathways by cytokines, growth factors, interleukins, binding proteins and pro-inflammatory molecules.
- Stimulation-dependent, observable changes in proliferation, bronchial epithelial permeability, crosslinking of membrane glycoproteins and cell surface adhesion molecules. Drug discovery cell screening for in vitro assay of compounds, or to extend and confirm high-throughput work done in cell lines.
- Clinical focused discoveries leveraging HBEpC include therapeutics to suppress tumor gene transcription, apoptosis, inflammation, auto-immune disease and viral infection, while enhancing cell protection, repair and lifespan.
Characterization: Morphology consistent with epithelial origin, and positive for epithelial cell marker cytokeratin 18
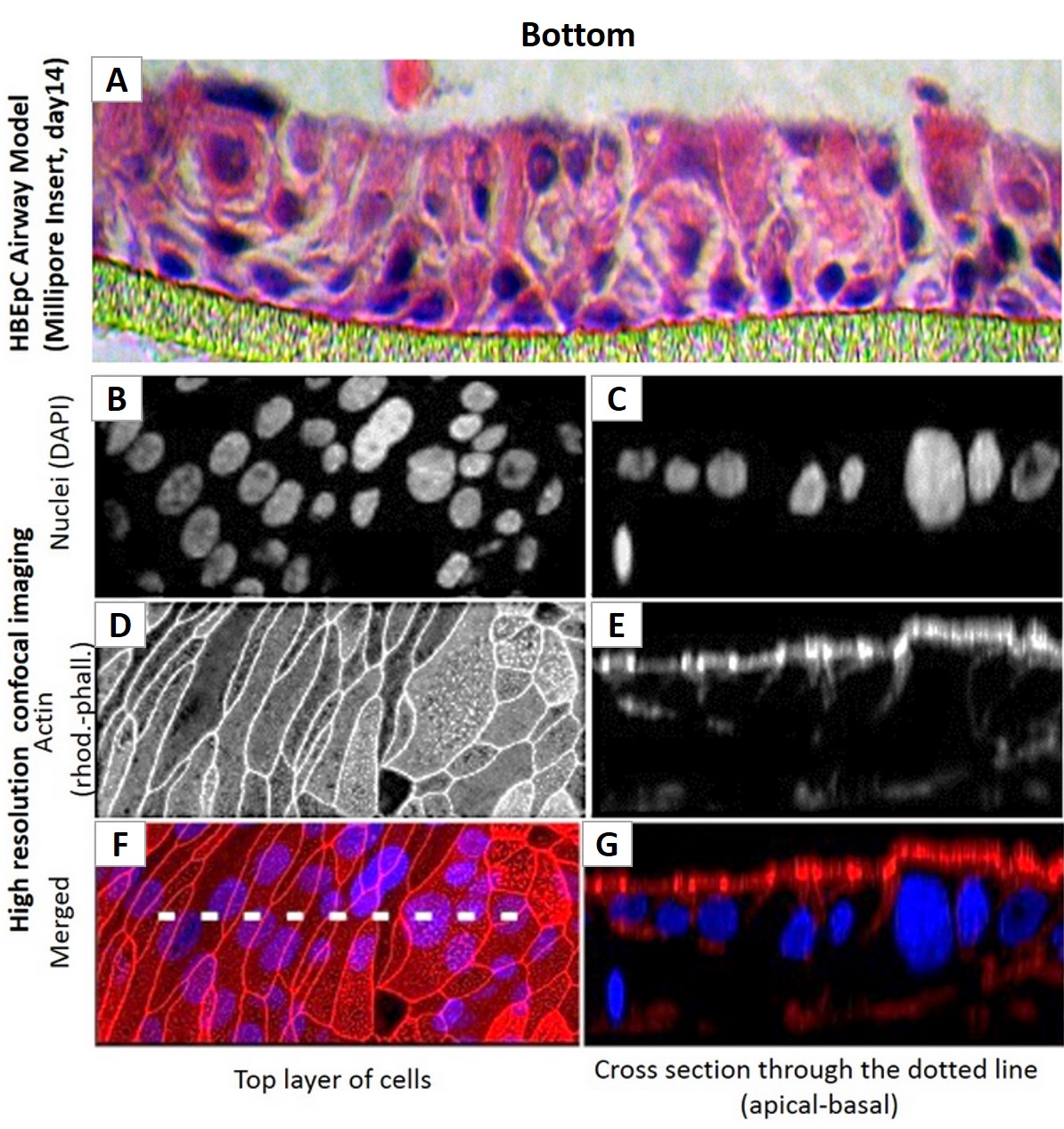
(Click to Enlarge) Human Bronchial Epithelial Cell (HBEpC)-based 3D airway tissue model, showing Millipore Insert, day 14 (A). High resolution confocal imaging (B–G) of the top cell layer (B,D,F) or a cross section (C,E,G). DAPI-labeled Nuclei (B&C), rhod-phall-labeled Actin (D&E) and merged images (F&G).
Details
| Tissue | Human bronchial epithelium: nondiseased, Asthma, COPD, or Type 2 Diabetes |
| QC | No bacteria, yeast, fungi, mycoplasma, virus |
| Bioassay | Attach, spread, proliferate in Growth Med |
| Cryovial | 500,000 HBEpC (1st passage) frozen in Basal Med w/ 10% FBS, 10% DMSO |
| Kit | Cryovial frozen HBEpC (502-05a), Gr Med (511-500), Subculture Rgnt Kit (090K) |
| Proliferating | Shipped in Gr Med, 3rd psg (flasks or plates) |
| Doublings | At least 16 |
| Applications | Laboratory research use only (RUO). Not for human, clinical, diagnostic or veterinary use. |
Products
| Product | Size | CAT.# | Price | Quantity |
|---|---|---|---|---|
| Cryopreserved Human Bronchial Epithelial Cell Total Kit, adult: 5x10^5 Cells (Adult), Medium & Subculture Reagents (See Details tab for specifics) | Size: 1 Kit | CAT.#: 502K-05a | Price: $1,049.00 | |
| Cryopreserved Bronchial Epithelial Cells (HBEpC), adult: Frozen HBEpC (5x10^5) | Size: 1 Cryovial | CAT.#: 502-05a | Price: $845.00 | |
| Cryopreserved Human Bronchial Epithelial Cell with Asthma Total Kit, adult: 5x10^5 Cells (from donor with Asthma, Adult), Medium & Subculture Reagents (See Details tab for specifics) | Size: 1 Kit | CAT.#: 502ASK-05a | Price: $1,279.00 | |
| Cryopreserved Bronchial Epithelial Cells, Asthma (HBEpC-AS), adult: Frozen HBEpC-AS from donor with Asthma (5x10^5) | Size: 1 Cryovial | CAT.#: 502AS-05a | Price: $1,075.00 | |
| Cryopreserved Human Bronchial Epithelial COPD Cell Total Kit, adult: 5x10^5 Cells (from donor with COPD, Adult), Medium & Subculture Reagents (See Details tab for specifics) | Size: 1 Kit | CAT.#: 502COPDK-05a | Price: $1,379.00 | |
| Cryopreserved Bronchial Epithelial Cells, COPD (HBEpC-COPD), adult: Frozen HBEpC-COPD from donor with Chronic Obstructive Pulmonary Disease (5x10^5) | Size: 1 Cryovial | CAT.#: 502COPD-05a | Price: $1,175.00 | |
| Proliferating Bronchial Epithelial Cells (HBEpC), adult: Actively growing, dividing cells in medium | Size: T-25 Flask | CAT.#: 503-25a | Price: $845.00 | |
| Proliferating Bronchial Epithelial Cells (HBEpC), adult: Actively growing, dividing cells in medium | Size: T-75 Flask | CAT.#: 503-75a | Price: $1,035.00 | |
| Proliferating Bronchial Epithelial Cells (HBEpC), adult: Actively growing, dividing cells in medium | Size: 24 Well | CAT.#: 503-24Wa | Price: $1,035.00 | |
| Proliferating Bronchial Epithelial Cells (HBEpC), adult: Actively growing, dividing cells in medium | Size: 96 Well | CAT.#: 503-96Wa | Price: $1,155.00 |
Related Products
| Product | Size | CAT.# | Price | Quantity |
|---|---|---|---|---|
| HBEpC/HTEpC Growth Medium: All-in-one ready-to-use. Cat# 511-500 fully supports cell growth on its own. Retinoic acid (RA) is a optional component; one vial of RA goes into 500ml media. Some researchers may elect to include RA if studies include cell differentiation or pseudo-stratification. | Size: 500 ml | CAT.#: 511-500 | Price: $145.00 | |
| HBEpC/HTEpC Growth Medium Kit: Basal medium & growth supplement sold together packaged separately | Size: 1 Kit | CAT.#: 511K-500 | Price: $154.00 | |
| HBrEpC/HTrEpC Basal Medium: Without growth supplement (GS). Add GS before use. | Size: 500 ml | CAT.#: 510-500 | Price: $92.00 | |
| HBEpC/HTEpC Growth Supplement: Added to Basal Medium to create Growth Medium | Size: 5 ml | CAT.#: 511-GS | Price: $88.00 | |
| HBEpC/HTEpC Differentiation Medium: Promotes cells to change from one type to another, more specialized | Size: 250 ml | CAT.#: 511D-250 | Price: $148.00 | |
| HBEpC/HTEpC Growth Medium wo Antibiotics: Growth medium without antibiotics | Size: 500 ml | CAT.#: 511A-500 | Price: $154.00 |
Extended Family Products
| Product | Size | CAT.# | Price | Quantity |
|---|---|---|---|---|
| 3-D Airway Model (Not available for international orders): HBEpC differentiated into pseudostriated epithelium on PCF sitting inserts. Available only in the US. Approximate turnaround time 4-6 weeks. | Size: 12 Sitting Inserts | CAT.#: 502-3D-12 | Price: $807.00 | |
| 3-D Airway Model (Not available for international orders): HBEpC differentiated into pseudostriated epithelium on PCF sitting inserts. Available only in the US. Approximate turnaround time 4-6 weeks. | Size: 24 Sitting Inserts | CAT.#: 502-3D-24 | Price: $1,422.00 | |
| 3-D Airway Model : Cells, Media, Reagents & Inserts (See Details tab for specifics) | Size: 1 Kit | CAT.#: 502K-3D | Price: $769.00 | |
| Monoclonal IL-1 Receptor Antagonist Antibody: Monoclonal IL-1 Receptor Antagonist Antibody | Size: 100 ul | CAT.#: CB19846 | Price: $302.00 | |
| Freezing Medium: For general cryopreservation of most primary cells. Contains FBS & DMSO. | Size: 50 ml | CAT.#: 040-50 | Price: $54.00 | |
| Cytofect Epithelial Cell Transfection Kit (124 x 24-Wells): 124 x 24-Well Rxns | Size: 1 Kit | CAT.#: TF102K | Price: $512.00 | |
| Cytofect Epithelial Cell Transfection Sample Kit (25 x 24-Wells): 25 x 24-Well Rxns | Size: 1 Sample Kit | CAT.#: TF102KS | Price: $68.00 | |
| Human Epidermal Growth Factor (EGF): Human Epidermal Growth Factor | Size: 100 ug | CAT.#: RP1026-100 | Price: $86.00 | |
| Human Epidermal Growth Factor (EGF): Human Epidermal Growth Factor | Size: 500 ug | CAT.#: RP1026-500 | Price: $194.00 | |
| Human Epidermal Growth Factor (EGF): Human Epidermal Growth Factor | Size: 1000 ug | CAT.#: RP1026-1000 | Price: $264.00 | |
| Bronchial Epithelial Cell RNA (HBEpC RNA), Adult: Total RNA prepared from Human Bronchial Epithelial Cells, adult | Size: 10 ug | CAT.#: 502-R10a | Price: $398.00 | |
| Bronchial Epithelial Cell RNA (HBEpC RNA), Adult: Total RNA prepared from Human Bronchial Epithelial Cells, adult | Size: 25 ug | CAT.#: 502-R25a | Price: $796.00 | |
| Human IL-1 alpha ELISA Kit: Human Interleukin-1 alpha ELISA Kit | Size: 96 wells | CAT.#: CL0389 | Price: $500.00 | |
| Human Interleukin-1-Alpha (IL-1 alpha): Human Interleukin-1-alpha | Size: 10 ug | CAT.#: RP1173-10 | Price: $194.00 | |
| Human Interleukin-1-Alpha (IL-1 alpha): Human Interleukin-1-alpha | Size: 100 ug | CAT.#: RP1173-100 | Price: $624.00 | |
| Human Interleukin-1-Alpha (IL-1 alpha): Human Interleukin-1-alpha | Size: 1000 ug | CAT.#: RP1173-1000 | Price: $5,327.00 | |
| Human IL-1 beta ELISA Kit: Human Interleukin-1 beta ELISA Kit | Size: 96 wells | CAT.#: CL0392 | Price: $587.00 | |
| Human Lung RNA: Total RNA prepared from human lung tissue | Size: 50 ug | CAT.#: 1H40-50 | Price: $228.00 | |
| Human Lung RNA: Total RNA prepared from human lung tissue | Size: 250 ug | CAT.#: 1H40-250 | Price: $851.00 | |
| Subculture Reagent Kit: 100 ml each of HBSS, Trypsin/EDTA & Trypsin Neutralizing Solution | Size: 1 Kit | CAT.#: 090K | Price: $69.00 | |
| Human EGF, Animal-Free: Human Epidermal Growth Factor, Animal-Free | Size: 100 ug | CAT.#: RP1026AF-100 | Price: $95.00 | |
| Human EGF, Animal-Free: Human Epidermal Growth Factor, Animal-Free | Size: 500 ug | CAT.#: RP1026AF-500 | Price: $213.00 | |
| Human EGF, Animal-Free: Human Epidermal Growth Factor, Animal-Free | Size: 1000 ug | CAT.#: RP1026AF-1000 | Price: $290.00 |
Resources/Documents
Citations
Publications
2017
Shah, F., Stepan, A.F., O'Mahony, A., Velichko, S., Folias, A.E., Houle, C., Shaffer, C.L., Marcek, J., Whritenour, J., Stanton, R. and Berg, E.L., 2017. Mechanisms of Skin Toxicity Associated with Metabotropic Glutamate Receptor 5 Negative Allosteric Modulators.Cell Chemical Biology, 858-869.e5.
Chakraborty, S., V. Castranova, M. Perez and G. Piedimonte. 2017. Nanoparticles-induced apoptosis of human airway epithelium is mediated by proNGF/p75NTR signaling. J Toxicol & Env Hlth, Pt A, 80:53-68.
2016
Hiraku, Y., F. Guo, N. Ma, T. Yamada, S. Wang, S. Kawanishi and M. Murata. 2016. Multi-walled carbon nanotube induces nitrative DNA damage in human lung epithelial cells via HMGB1-RAGE interaction and Toll-like receptor 9 activation. Particle & Fibre Toxicol, 13:16.
Mungunsukh, O., Y. Lee, D. Bottaro and R. Day. 2016. The Hepatocyte Growth Factor Isoform NK2 Activates Motogenesis and Survival but not Proliferation due to Lack of Akt Activation. Cellular Signaling, 28:1114-1123.
Othumpangat, S., J. Noti, C. McMillen and D. Beezhold. 2016. ICAM-1 regulates the survival of influenza virus in lung epithelial cells during the early stages of infection. Virology, 487:85-94.
Perez, D. and H. Chen. 2016. Methods and Compositions for in vivo Immune Stimulation and Antigen Production. Patent Application US 20160022807 A1.
2015
Burns, T., A. Ali, and D. Matesic. 2015. Comparative Effects of 4-Phenyl-3-Butenoic Acid and Vorinostat on Cell Growth and Signaling. Anticancer Research, 35: 775-784.
Li, J., J. Guan X. Long and X. Xiang. 2015. Endothelin-1 Upregulates the Expression of High Mobility Group Box 1 in Human Bronchial Epithelial Cells. Pharmacology, 96:144-150.
Othumpangat, S., J. Noti, C. McMillen, and D. Beezhold. 2015. ICAM-1 regulates the survival of influenza virus in lung epithelial cells during the early stages of infection. Virology, 487:85-94.
2014
Chen, H., M. Angel, W. Li, C. Finch, A. Gonzalez, T. Sutton, J. Santos, and D. Perez. 2014. All-in-One Bacmids: an Efficient Reverse. J Virol, 88:10013.
Fischer, D., R. Janssen, M. Roseboom, A. Scaffidi, and M. Tessari. 2014. Methods for identifying and compounds useful for increasing the functional activity and cell surface expression of CF-associated mutant cystic fibrosis transmembrane conductance regulator. Patent US 8765376 B2.
Haselmayer, P., M. Camps, M. Muzerelle, S. El_bawab, C. Waltzinger, L. Bruns, N. Abla, M. Polokoff, C. Jond-necand, and M. Gaudet. 2014. Characterization of novel PI3Kδ inhibitors as potential therapeutics for SLE and lupus nephritis in pre-clinical studies. Name: Frontiers in Immunology. 5:233.
Hattori, S., K. Kojima, K. Minoshima, M. Yamaha, M. Horie, T. Sawamura, A. Kikuchi, and T. Deguchi. 2014. Detection of bladder cancer by measuring CD44v6 expression in urine with real-time quantitative reverse transcription polymerase chain reaction. Urology, 83:1443.e9-1443.e15.
Gaillard, and H. Ji. 2014. Characterization of novel PI3Kδ inhibitors as potential therapeutics for SLE and lupus nephritis in pre-clinical studies. Front Immunol, 5:233.
Nasreen, N., L. Gonzalves, S. Peruvemba and K. Mohammed. 2014. Fluticasone furoate is more effective than mometasone furoate in restoring tobacco smoke inhibited SOCS-3 expression in airway epithelial cells. Intl Immunopharmacol, 19:153-160.
Prakash, S., S. Agrawal, H. Vahed, M. Ngyuen, L. BenMohamad, S. Gupta and A. Agrawal. 2014. Dendritic cells from aged subjects contribute to chronic airway inflammation by activating bronchial epithelial cells under steady state. Mucosal Immunol, 7:1386-1394.
Tripathi, B., X. Qian, P. Mertins, D. Wang, A. Papageorge, S. Carr, and D. Lowy. 2014. CDK5 is a major regulator of the tumor suppressor DLC1. JCB, 207:627-642.
Voiles, L., D. Lewis, L. Han, I. Lupov, T. Lin, M. Robertson, I. Petrache and H. Chang. 2014. Overexpression of type VI collagen in neoplastic lung tissues. Oncology Reports, 32:1897-1904.
2012
Abdullah, L., C. Wolber, M. Kesimer, J. Sheehan, and C.W. Davis. 2012. Studying Mucin Secretion from Human Bronchial Epithelial Cell Primary Cultures. In Mucins. Vol. 842. M.A. McGuckin and D.J. Thornton, editors. Humana Press. 259-277.
Narisawa-Saito, M., Y. Inagawa, Y. Yoshimatsu, K. Haga, K. Tanaka, N. Egawa, S. Ohno, H. Ichikawa, T. Yugawa, M. Fujita and T. Kiyono. 2012. A critical role of MYC for transformation of human cells by HPV16 E6E7 and oncogenic HRAS. Carcinogenesis, 33:910-917.
Nasreen, N., N. Khodayari, B. Sukka-Ganesh, S. Peruvemba, and K.A. Mohammed. 2012. Fluticasone propionate and Salmeterol combination induces SOCS-3 expression in airway epithelial cells. International Immunopharmacology. 12:217-225.
Othumpangat, S., M. Regier and G. Piedimonte. 2012. Nerve growth factor modulates human rhinovirus infection in airway epithelial cells by controlling ICAM-1 expression. Am J. Physiol – Lung Cellular & Molec Physiol, 302:L1057-L1066.
Othumpangat, S., C. Walton, and G. Piedimonte. 2012b. MicroRNA-221 Modulates RSV Replication in Human Bronchial Epithelium by Targeting NGF Expression. PloS one. 7:e30030.
2010
Berg, E.L., J. Yang, J. Melrose, D. Nguyen, S. Privat, E. Rosler, E.J. Kunkel, and S. Ekins. 2010. Chemical target and pathway toxicity mechanisms defined in primary human cell systems. Journal of Pharmacological and Toxicological Methods. 61:3-15.
Lung, J., K.-J. Liu, J.-Y. Chang, S.-J. Leu, and N.-Y. Shih. 2010. MBP-1 is efficiently encoded by an alternative transcript of the ENO1 gene but post-translationally regulated by proteasome-dependent protein turnover. FEBS Journal. 277:4308-4321.
Maier, K.G., X. Han, B. Sadowitz, K.L. Gentile, F.A. Middleton, and V. Gahtan. 2010. Thrombospondin-1: a proatherosclerotic protein augmented by hyperglycemia. Journal of Vascular Surgery. 51:1238-1247.
Mungunsukh, O., A.J. Griffin, Y.H. Lee, and R.M. Day. 2010. Bleomycin induces the extrinsic apoptotic pathway in pulmonary endothelial cells. American Journal of Physiology - Lung Cellular and Molecular Physiology. 298:L696-L703.
Tai, H.Y., M.F. Tam, H. Chou, D.W. Perng, and H.D. Shen. 2010. Pen ch 13 Major Fungal Allergen Decreases CD44 Expression in Human Bronchial Epithelial Cells. International Archives of Allergy and Immunology. 153:367-371.
2009
Houck, K.A., D.J. Dix, R.S. Judson, R.J. Kavlock, J. Yang, and E.L. Berg. 2009. Profiling Bioactivity of the ToxCast Chemical Library Using BioMAP Primary Human Cell Systems. Journal of biomolecular screening. 14:1054-1066.
Schembri, F., S. Sridhar, C. Perdomo, A.M. Gustafson, X. Zhang, A. Ergun, J. Lu, G. Liu, X. Zhang, J. Bowers, C. Vaziri, K. Ott, K. Sensinger, J.J. Collins, J.S. Brody, R. Getts, M.E. Lenburg, and A. Spira. 2009. MicroRNAs as modulators of smoking-induced gene expression changes in human airway epithelium. PNAS. doi: 10.1073/pnas.0806383106.
Song, H., H. Wan, Y. Araya, and D. Perez. 2009. Partial direct contact transmission in ferrets of a mallard H7N3 influenza virus with typical avian-like receptor specificity. Virology Journal. 6:126.
Tanaka, H., T. Fukushima, K. Yorita, M. Kawaguchi, and H. Kataoka. 2009. Tissue injury alters the site of expression of hepatocyte growth factor activator inhibitor type 1 in bronchial epithelial cells. Human Cell. 22:11-17.
Tumpey, and F. Fang. 2009. Novel Pandemic Influenza A(H1N1) Viruses Are Potently Inhibited by DAS181, a Sialidase Fusion Protein. PloS one. 4:e7788.
2010
Berg, E.L., J. Yang, J. Melrose, D. Nguyen, S. Privat, E. Rosler, E.J. Kunkel, and S. Ekins. 2010. Chemical target and pathway toxicity mechanisms defined in primary human cell systems. Journal of Pharmacological and Toxicological Methods. 61:3-15.
Lee, Y.H., O. Mungunsukh, R.L. Tutino, A.P. Marquez, and R.M. Day. 2010. Angiotensin-II-induced apoptosis requires regulation of nucleolin and Bcl-xL by SHP-2 in primary lung endothelial cells. Journal of cell science. 123:1634-1643.
Lung, J., K.-J. Liu, J.-Y. Chang, S.-J. Leu, and N.-Y. Shih. 2010. MBP-1 is efficiently encoded by an alternative transcript of the ENO1 gene but post-translationally regulated by proteasome-dependent protein turnover. FEBS Journal. 277:4308-4321.
Mungunsukh, O., A.J. Griffin, Y.H. Lee, and R.M. Day. 2010. Bleomycin induces the extrinsic apoptotic pathway in pulmonary endothelial cells. American Journal of Physiology - Lung Cellular and Molecular Physiology. 298:L696-L703.
Tai, H.Y., M.F. Tam, H. Chou, D.W. Perng, and H.D. Shen. 2010. Pen ch 13 Major Fungal Allergen Decreases CD44 Expression in Human Bronchial Epithelial Cells. International Archives of Allergy and Immunology. 153:367-371.
2008
Lee, Y.H., Y.J. Suzuki, A.J. Griffin, and R.M. Day. 2008. Hepatocyte growth factor regulates cyclooxygenase-2 expression via β-catenin, Akt, and p42/p44 MAPK in human bronchial epithelial cells. American Journal of Physiology. 294:L778-L786.
2007
Haga, K., S.-i. Ohno, T. Yugawa, M. Narisawa-Saito, M. Fujita, M. Sakamoto, D.A. Galloway, and T. Kiyono. 2007. Efficient immortalization of primary human cells by p16INK4a-specific short hairpin RNA or Bmi-1, combined with introduction of hTERT. Cancer science. 98:147-154.
Handa, K., T. Yugawa, M. Narisawa-Saito, S.-i. Ohno, M. Fujita, and T. Kiyono. 2007. E6AP-Dependent Degradation of DLG4/PSD95 by High-Risk Human Papillomavirus Type 18 E6 Protein. Journal of Virology. 81:1379-1389.
Horvath, G., E.S. Mendes, N. Schmid, A. Schmid, G.E. Conner, M. Salathe, and A. Wanner. 2007. The effect of corticosteroids on the disposal of long-acting β2-agonists by airway smooth muscle cells. Journal of Allergy and Clinical Immunology. 120:1103-1109.
2006
Tai, H., M. Tam, H. Chou, H. Peng, S. Su, D. Perng, and H. Shen. 2006. Pen ch 13 allergen induces secretion of mediators and degradation of occludin protein of human lung epithelial cells. 61:382-388.
2005
Lee, S.C., J.Y. Hsu, L.S. Fu, J.J. Chu, S.J. Fan, and C.S. Chi. 2005. Comparison of the activities of granulocyte-macrophage colony-stimulating factor and interleukin-8 secretion between two lung epithelial cell lines. J. microbial., immunol.& infection. 38:327-331.
Look, D.C., L.L. Stoll, S.A. Romig, A. Humlicek, B.E. Britigan, and G.M. Denning. 2005. Pyocyanin and Its Precursor Phenazine-1-Carboxylic Acid Increase IL-8 and Intercellular Adhesion Molecule-1 Expression in Human Airway Epithelial Cells by Oxidant-Dependent Mechanisms. The Journal of Immunology. 175:4017-4023.
2004
Kilani, M., K. Mohammed, N. Nasreen, R. Tepper, and V. Antony. 2004a. RSV Causes HIF-1α Stabilization via NO Release in Primary Bronchial Epithelial Cells. Inflammation. 28:245-251.
Kilani, M.M., K.A. Mohammed, N. Nasreen, J.A. Hardwick, M.H. Kaplan, R.S. Tepper, and V.B. Antony. 2004b. Respiratory syncytial virus causes increased bronchial epithelial permeability*. CHEST Journal. 126:186-191.
2003
O'Malley, Y.Q., K.J. Reszka, G.T. Rasmussen, M.Y. Abdalla, G.M. Denning, and B.E. Britigan. 2003. The Pseudomonas secretory product pyocyanin inhibits catalase activity in human lung epithelial cells. American Journal of Physiology. 285:L1077-L1086.
