Product Sheet CL0386
Description
BACKGROUND Insulin-like growth factor binding protein-3 (IGFBP-3) is one of a family of six IGFBPs, multifunctional proteins that transport and stabilize IGFs in the circulation, modulate the effects of IGF on a variety of cellular functions, and may also regulate cells by IGF independent mechanisms. Initially purified from human plasma, IGFBP-3, a 40–45 kDa glycoprotein, is by far the most abundant IGFBP in the circulation, where it has a central role in regulating IGF bioavailability to the tissues by forming heterotrimeric complexes with IGF-I or IGF-II and an 85 kDa glycoprotein, the acid labile subunit. IGFBP-3 is also active in the cellular environment.1 It was showed that IGFBP-3 production is potently induced by TGF-beta. Moreover, it was also shown that the induction of IGFBP-3 in the mode of action of a variety of growth inhibitory proteins and agents, including p53, retinoic acid, vitamin D, anti-oestrogens, and TNF-alpha. The antiproliferative activity of IGFBP-3 may contribute to its anti-oncogenic effects.2
The effects of IGFBP-3 on IGF dependent cellular functions are complex, with both stimulatory and inhibitory actions reported, even within the one cell type. Several studies with cells lacking the type I IGF receptor have revealed that IGFBP-3 can be inhibitory to cell growth even in the absence of IGF signaling, raising the possibility of truly IGF independent effects of IGFBP-3. To date, no cell surface IGFBP-3 receptor that initiates an intracellular signaling cascade has been identified. It was shown that IGFBP-3 can stimulate phosphorylation of the cell surface receptor TGF-betaRI as well as phosphorylation of the TGF-beta signaling intermediates Smad2 and Smad3. Moreover, IGFBP-3 activates the promoter of the TGF-beta-responsive gene, PAI-1, which encodes plasminogen activator inhibitor-1 (PAI-1). PAI-1 blocks the activation of plasminogen to plasmin, a protease that causes partial fragmentation of IGFBP-3, thus limiting its ability to inhibit IGF action.3
The effects of IGFBP-3 on IGF dependent cellular functions are complex, with both stimulatory and inhibitory actions reported, even within the one cell type. Several studies with cells lacking the type I IGF receptor have revealed that IGFBP-3 can be inhibitory to cell growth even in the absence of IGF signaling, raising the possibility of truly IGF independent effects of IGFBP-3. To date, no cell surface IGFBP-3 receptor that initiates an intracellular signaling cascade has been identified. It was shown that IGFBP-3 can stimulate phosphorylation of the cell surface receptor TGF-betaRI as well as phosphorylation of the TGF-beta signaling intermediates Smad2 and Smad3. Moreover, IGFBP-3 activates the promoter of the TGF-beta-responsive gene, PAI-1, which encodes plasminogen activator inhibitor-1 (PAI-1). PAI-1 blocks the activation of plasminogen to plasmin, a protease that causes partial fragmentation of IGFBP-3, thus limiting its ability to inhibit IGF action.3
REFERENCES
1. Renehan, A.G. et al: Endocr Relat Cancer. 13:273-8, 2006
2. Burger, A.M. et al: Eur J Cancer 41:1515-27, 2005
3. Kuemmerle, J.F. et al: Am J Physiol Gastrointest Liver Physiol.;287:G795-802, 2004
2. Burger, A.M. et al: Eur J Cancer 41:1515-27, 2005
3. Kuemmerle, J.F. et al: Am J Physiol Gastrointest Liver Physiol.;287:G795-802, 2004
Products are for research use only. They are not intended for human, animal, or diagnostic applications.
Details
Cat.No.: | CL0386 |
Target Protein Species: | Human |
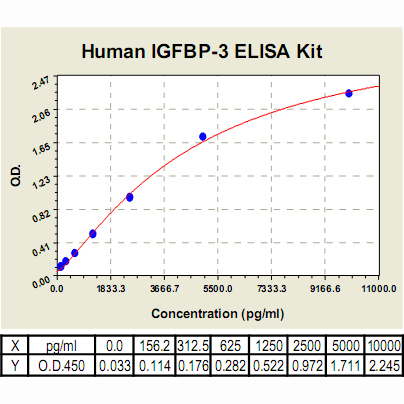
Range: | 156.2 pg/ml – 10000pg/ml |
Specificity: | No detectable cross-reactivity with any other cytokines |
Storage: | Store at 4°C. Use within 6 months. |
ELISA Kits are based on standard sandwich enzyme-linked immunosorbent assay technology. Freshly prepared standards, samples, and solutions are recommended for best results.
Products
| Product | Size | CAT.# | Price | Quantity |
|---|---|---|---|---|
| Human IGFBP-3 ELISA Kit: Human Insulin-Like Growth Factor-Binding Protein 3 ELISA Kit | Size: 96 wells | CAT.#: CL0386 | Price: $484.00 |
Resources/Documents
Publications
2015
Miljus, G., V. Malenkovic, B. Dukanovic, N. Kolundzic, and O. Nedic. 2015. IGFBP-3/transferrin/transferrin receptor 1 complexes as principal mediators of IGFBP-3 delivery to colon cells in non-cancer and cancer tissues. Experimental and Molecular Pathology, 98:431-438.