Anti-S100B: Mouse S100B Antibody
Mouse S100B Antibody: Mouse S100B Antibody
Size: 100 ul
Price: $501.00
Description
S100B is a member of the S100 family of proteins. The highest level of expression of the S100B protein is in the brain and is found primarily in the cytoplasm of astrocytes. Results of in vitro studies suggest a variety of intracellular functions of S100B, including cell growth, cell structure, energy metabolism, and calcium homeostasis. S100B is secreted from astrocytes, suggesting that it might also have extracellular functions. Exogenous S100B increases intracellular calcium concentrations in both cultured neurons and astrocytes. Elevated neuronal calcium might affect calcium-dependent processes involved in synaptic plasticity.2 In addition S100B is involved in regulation of cell cycle progression and cell differentiation. A Ca2+ dependent conformational change in dimeric S100B (beta-beta) is required for it to bind p53 and inhibit phosphorylation of this tumor suppressor in its C-terminal negative regulatory domain. Moreover, S100B and p53 interaction not only induces total inhibition of p53 oligomerization but also promotes disassembly of the p53 oligomers.3
2. Nishiyama, H. et al: Proc. Natl. Acad. Sci. USA 99:4037-42, 2002
3. Rust, R.R. et al: Nature Struct. Biol. 7:570-4, 2000
Details
| Cat.No.: | CP10223 |
| Antigen: | Purified recombinant human S100B proteins expressed in E. coli. |
| Isotype: | Mouse IgG1 |
| Species & predicted species cross- reactivity ( ): | Human |
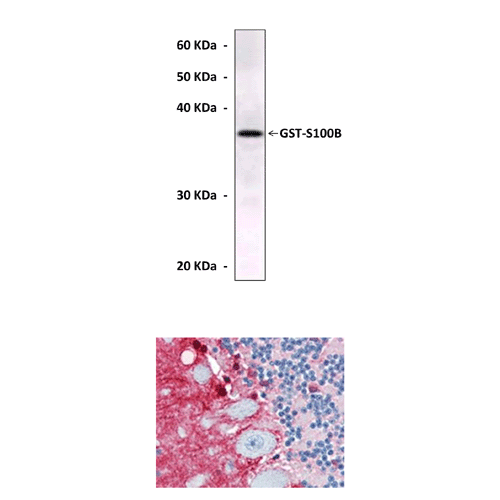
| Applications & Suggested starting dilutions:* | WB 1:1000 IP n/d IHC 1:200 ICC n/d FACS n/d |
| Predicted Molecular Weight of protein: | 11 kDa |
| Specificity/Sensitivity: | Detects S100B proteins without cross-reactivity with other family members. |
| Storage: | Store at -20°C, 4°C for frequent use. Avoid repeated freeze-thaw cycles. |
*Optimal working dilutions must be determined by end user.