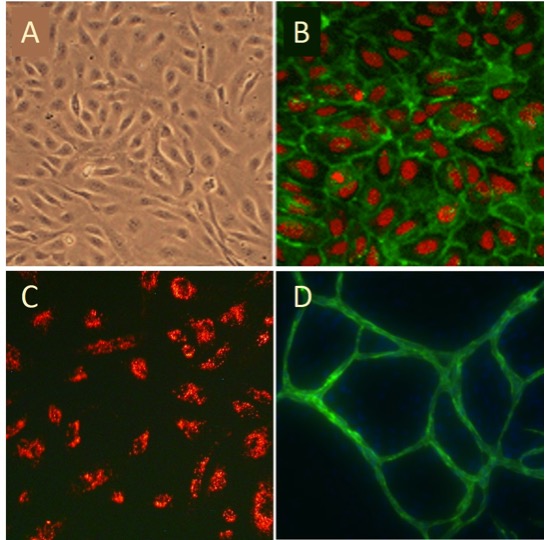
Human Umbilical Vein Endothelial Cells: HUVEC
Description
Human Umbilical Vein Endothelial Cells (HUVEC) provide a classic model system to study many aspects of endothelial function and disease, such as normal, abnormal and tumor-associated angiogenesis, oxidative stress, hypoxia and inflammation related pathways in endothelia under normal and pathological conditions, cardiovascular-related complications associated with various diseases, mode of action and cardiovascular protection effects of various compounds, etc.
Select HUVEC lots have been tested to demonstrate stimulation-dependent angiogenesis and key endothelial cell signaling pathways (phosphorylation of VEGFR2). More information about pre-screened endothelial cells can be found on the Pre-Screened Endothelial Cell Product Page.
HUVEC from Cell Applications, Inc. have been utilized in numerous research publications, for example, to:
- Understand anti-inflammatory properties of HDL
- Develop scaffolds for tissue engineering
- Characterize endothelial cell response to stress and discover mechanisms that protect mitochondria against oxidative stress
- Demonstrate that prolonged hypoxia induces MMP-1 expression, EC migration and angiogenesis
- Show that Alzheimer’s β-amyloid peptide exhibits anti-angiogenic properties
- Demonstrate for the first time that mature miRNA can control gene expression in a cell where it is neither transcribed nor processed
- Show that abnormal matrix composition characteristic for systemic sclerosis leads to impaired vascular function and angiogenesis
- Discover novel mechanisms of inhibiting tumor angiogenesis and metastasis, by limiting EC migration and proliferation, as well as the expression of growth factors and enzymes
- Develop a bubble lyposome based system that enables therapeutic miRNA delivery
- Demonstrate that commercially used flame retardants cause oxidative stress
- Show that hyperglycemia leads to endothelial dysfunction, partially due to depletion of antioxidants, and that regaining glycemic control normalizes growth factor levels in ischemia and improves perfusion recovery
- Show that inhibition of aldose reductase inhibits migration and formation of capillary-like structures by endothelial cells
- Investigate effects of molecular mobility of the outmost material surfaces on cellular adhesion and organization
- Identify signaling differences in cells cultured on different ECM components.
- Investigate mechanisms of cardiovascular protection exerted by bioactive plant and fungal components
- Show that in low doses coumaric acid and resveratrol provide beneficial effects and protect endothelial cells from reactive oxygen species
- Show that combined treatment with melatonin and atorvastatin reversed damage to EC, partly by reducing free radical generation and lipid peroxidation
- Discover that deferoxamine (DFO), an iron-chelating agent, mitigates deleterious effects of radiation on angiogenesis
- Study the differential effects of wood smoke and diesel exhaust particles, as well as carbon nanotubes on inducing oxidative stress and production of cytokines and adhesion molecules in endothelia
Details
| Tissue | Healthy human umbilical vein | |
|---|---|---|
| QC | No bacteria, yeast, fungi, mycoplasma, virus | |
| Character | Factor VIII-related Ag, DiI-Ac-LDL uptake. S-HUVEC are select HUVEC lots that have been tested positive for VEGFR2 pathway activation following stimulation by VEGF. | |
| Bioassay | Attach, spread, proliferate in Growth Med | |
| Cryovial | 500,000 HUVEC (primary culture) in Bas Med w/ 10% FBS, 10% DMSO | |
| Kit | Cryovial frozen HUVEC (200-05n), Growth Medium (211-500), Subculture Rgnt Kit (090K) | |
| Proliferating | Shipped in Tsfr Med, 1st psg (flasks or plates) | |
| Doublings | At least 16 | |
| Applications | Laboratory research use only (RUO). Not for human, clinical, diagnostic or veterinary use. |
Resources
FAQs
Need More Help?
Visit our comprehensive FAQ page for detailed answers to common questions.
Need More Help?
Visit our comprehensive FAQ page for detailed answers to common questions.
Primary Cell FAQs